Apart from the incubators and the Class II hoods, perhaps one of the most overlooked but important pieces of equipment in the cell culture lab is the haemocytometer. For accurate cell-seeding experiments and splitting cells, this thick glass slide is an essential tool for everyone working with live cells.
There are a number of different haemocytometers on the market and each one has a different grid pattern as well as different recommended uses.
Louis-Charles Malassez (1842-1909) was a French histologist and anatomist and is the person credited for inventing the haemocytometer. As the name suggests, this device was originally intended for the quantitative counting of blood cells.
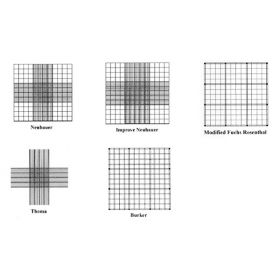
The most frequently used haemocytometer is the Neubauer (or ‘Improved Neubauer’) chamber. Other haemocytometers include the Burker, Thoma and Fuchs-Rosenthal. Most of the haemocytometers are manufactured from crystal glass and generally measure 30 x 70 mm with a thickness of 4 mm. Two vertical lines are ground from the glass to define the counting area and the double cell counting chambers have a ground out ‘H’ shape. A selection of haemocytometers is available from Agar Scientific;
www.agarscientific.com/lm/slides-coverslips/haemacytometers
The Neubauer/Improved Neubauer haemocytometer
The cell counting area of the Neubauer measures 3 mm2 (with each of the main squares measuring 1 mm);

The grid is subdivided and the 16 squares at each of the four corners measure 500 mm2. In a Neubauer chamber, the central square consists of 16 smaller squares each one with the same width/height the corner squares. Each of the 16 central squares is further subdivided into 16 smaller squares. The glass covers for haemocytometers are specifically designed with regards to thickness and size (generally 22 mm2). Do not be tempted to use a coverslip as this may result in an inaccurate cell count. When the coverglass is placed over the counting area, this leaves a specific area for which to introduce the cells/liquid to be counted. The Neubauer chamber is designed to leave a gap of 100 mm between the top surface of the counting area and the bottom surface of the coverglass.
The Improved Neubauer has a slightly different grid pattern compared to the ‘old’ Neubauer chamber. The overall size of the central counting area is still the same, but the square in the central cross area are divided into 25 smaller squares, each one measuring 400 mm2;
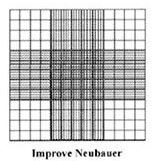
Each of the 25 squares in the centre of the cross grid are further subdivided into 16 smaller squares which measure 2.5 mm2(or 0.0025 mm2 as etched onto the surface of the Improved Neubauer slide). In other words, the central cross square contains 400 small squares.
The Neubauer chambers are designed with these grid patterns for the counting of blood cells (although, these chambers are suited for the counting of most mammalian cell lines/primary cells etc.). The larger squares at the four corners are designed for the counting of white blood cells whereas the central smaller squares are for counting red blood cells and platelets.
The Burker Haemocytometer
The Burker chamber has the same dimension of grid (and same depth) as the Neubauer slide;
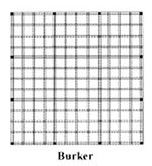
The main difference is that the central square is not sub-divided into the 16 or 25 smaller squares. The grid pattern runs across the whole counting area and the central square contains the same number of smaller squares (16) as the corner and side squares.
The Thoma Haemocytometer
The Thoma chamber has the same dimensions and depth as the Neubauer chamber, however, this haemocytometer does not have the four larger corner squares;
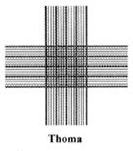
The Thoma haemocytometer seems to be the favoured counting chamber for microbiologists.
The Fuchs-Rosenthal Haemocytometer
This counting chamber is specifically designed for the counting of cells from cerebro-spinal fluid. The main difference (apart from the counting grid pattern) is that the distance between the coverslip and the chamber surface is 200 mm. This gives a larger volume of cells/liquid which can be counted and is also used for counting blood cells.
The grid pattern of the Fuchs Rosenthal is similar to the Burker chamber, except that the Fuchs Rosenthal haemocytometer is designed to accommodate twice as much volume compared to the other chambers mentioned above.
Counting cells using a haemocytometer
For this example, we will consider the counting of a sample of cells using an Improved Neubauer haemocytometer (although this can be applied to any of the haemocytometers above excluding the Thoma chamber).
Firstly, you’ll need to clean and prepare the chamber- the easiest way is with 70% ethanol. Dry the chamber (preferably with a lens cleaning tissue). You will then need to moisten the edges of the chamber and the most common way is with the exhaled breath with mouth fully open. Slide on the coverglass using gentle pressure and you should see something called ‘Newton’s Rings’ where the coverglass is in contact with the chamber (rings caused by an interference pattern).
- Prepare the cell suspension
The cells and fluid (such as culture medium, PBS etc.) should be well mixed to provide a homogenous sample. You can do this by gently pipetting the cells up and down a pipette tip or by gently agitating the flask/container. Don’t be tempted to use excessive pipetting or a vortex mixer as this may shear cells.
Quickly remove a volume of cells and transfer to an Eppendorf tube. Add an equal volume of trypan blue and gently mix by pipetting or by tapping the tube (note- this is an example using a 1:1 dilution, but you may need to adjust depending on the cell density). Using trypan blue will help to distinguish between dead (blue) cells and living (clear) cells.
- Loading and counting cells
- With a pipette, carefully draw up around 20 ml of the cell mixture. Place the pipette tip against the edge of the coverglass and slowly expel the liquid until the counting chamber is full. Capillary action will help to ensure that the counting chamber is full, but care should be taken not to overfill the chamber. A volume of 10 ml is sufficient to fill one counting chamber.
- Place the haemocytometer on the microscope stage. Using the 10X objective, focus on the grid lines. The general rule for counting of cells which fall on the edges of the squares is only to count those cells which are on the top and the left hand side grid lines. This rule ensures that you do not count the same cells twice.
- It is advisable to use a hand tally to keep count of cells. Count the number of cells in one of the corner squares (which are further separated into 16 squares). Count all of the clear cells within the squares and those touching the top and left hand grid lines. Another tip is to count in a ‘turret’ pattern, i.e., count along the top row of squares from left to right, down to the next square and count from right to left and so on until all the cells in the 16 small squares have been counted.
- Move to the next corner square and repeat until you have counted the four larger corner squares.
- The volume of liquid between the coverglass and the counting chamber is such that the number of cells which are counted in one set of 16 small squares is equivalent to the number of cells X 104/ml.
- Calculating the total number of cells
Add the four counts together and divide by four to give an average over the whole counting chamber.
Multiply by 2 to take into account the 1:1 dilution made when adding the trypan blue to the cell mixture.
The total count from 4 sets of 16 corner squares = (cells/mL x 104) x 4 squares from one hemocytometer grid.
In other words;
Total cells (X104)/ml = Total cells counted x (dilution factor/number of squares counted)
For example;
-Total cell count was 180
-Number of squares counted was 4
-100 ml of trypan blue was added to 100 ml of cell suspension
Therefore;
Total cells (X104)/ml = 180 x (2/4)
Which gives 90 X 104 cells/ml.
If you find that there are too many cells in your suspension, then simply increase the dilution factor of cells to trypan blue (but remember to take this into account in the final calculation of cell density).
For an accurate determination of the total number of cells, the number of cells in one of the large squares should be between 15 and 50.
AUTHOR: Martin Wilson