The method of Koehler Illumination is named after its’ inventor- August Koehler (1866-1948). When he published this technique in 1893, many of the sources of illumination for microscopy were reliant on natural light using mirrors, or chemical or gas lights. In addition, emulsion plates were being used for photomicroscopy which produced inconsistent results due to the long exposure times required.
History of Koehler illumination
The technique which Koehler published was entitled “A New System of Illumination for Photomicrographic Purposes” and printed in the Journal of Scientific Microscopy and Microscopic Technique. In this paper, he highlighted some of the problems which faced microscopists in the late 19th Century. Two of the main sources of illumination included zirconia light and Auer gas lights- both of which had a tendency to produce an unwanted ‘glare’ as well as being relatively inconsistent.
It was the Austrian scientist, Baron Carl Auer von Welsbach (1858-1929) whom the ‘Auer’ gas light was named after. The London Journal of Gas Lighting and Sanitary Water Supply claimed that the Auer gas lamp was “eminently suitable” for microscopy despite producing heat and carbonic acid. Auer is also credited for lighting many of the streets in Europe after patenting his gas mantle.
The other source of illumination (‘zirconia light’) was an intense chemical light which was produced by heating the element zirconium. In his paper, Koehler highlighted the problems of using zirconia light and Auer gas lamps, stating that “it is difficult or impossible to achieve uniform illuminations”. To have consistency in illumination (especially for photomicroscopy), Koehler realised that every single optical component of the microscope needed to be correctly aligned in relation to each other. By applying his technique to the main problems of inconsistency of light (and unwanted heat) he managed to work around these difficulties by making adjustments to the condensers and diaphragms of the microscope. He even went as far as using an opera glass in place of the convex lens!
Following his publication, in 1900 August Koehler was invited to work for the Carl Zeiss Company where he remained until 1945.
A foundation of consistent microscopic technique
More than 120 years after the initial publication of his technique, Koehler illumination is still widely used and also forms the foundation of a number of methods such as confocal, differential interference contrast (DIC) and phase contrast microscopy. It’s simply one of the most fundamental and important principles of good light microscopy! After you learn how to correctly set up a microscope for Koehler illumination, it will become second nature and will transform your images.
Despite this, throughout my years working in teaching and research labs, it is a technique which is still not fully appreciated or practised by those using microscopes. I have come across many senior scientist, professors and pathologists who are not even aware of this technique, let alone can set up the microscope for optimal illumination. However, this is not through ignorance- it’s another of those ‘taken for granted’ foundations of imaging which still doesn’t seem to be taught at the undergraduate level or many scientists simply don’t have the time to go back and read and learn the basics.
However, do you really want to end up with mediocre images after spending hours and days in histology- processing, embedding, sectioning and days spent optimising your immunohistochemistry and staining? Sure, if you just want to routinely check slides, then it’s simply a case of placing them on the microscope stage, logging into the PC and off you go.
Alternatively, once you learn how to do this, it takes less than a minute to set up the correct alignment. A correctly aligned microscope will;
– ensure that your specimen is evenly illuminated
– increase the contrast between areas of staining or between regions of interest in your sample (especially important for viewing non-stained live cells)
– be able to collect more imaging data
– increase the overall resolution of the microscope and produce a sharper final image.
If you are planning to use any of your final images for presentations (such as Powerpoint or posters) or if you need images for a research paper, then Koehler illumination is an absolute must. If you practice the simple five step method highlighted below it will soon second nature.
In addition to selecting the magnification required for your images, there are two other optical components with which you should be familiar with to set up for Koehler illumination.
1) The field diaphragm
In an upright microscope, the field diaphragm is usually located in the body or base of the instrument. If you are using an older, or simpler, microscope, it may just have an on/off switch for the light source and/or have an intensity control as part of the switch. Some older (or simpler) microscopes may just have an ‘on/off’ switch for the light source and there may be no field diaphragm to control the intensity of light (if this is the case, simply use the diaphragm of the sub-stage condenser- see below). On some instruments, the diaphragm may be present as a knurled ring around the light, or a separate wheel next to the illumination source. The purpose of the field diaphragm is to control the intensity of light which passes through the sub-stage condenser and on to the stage. Controlling the light results in a reduced glare in the final image as well as increasing the contrast across your specimen.
2) The sub-stage condenser
On an upright microscope the sub-stage condenser will be directly under the stage. Alternatively, in an inverted configuration, the condenser is located above the stage but before the light source and field diaphragm. The sub-stage condenser has a set of focus wheels which are similar (and usually smaller) than the main focus of the microscope. The purpose of this optical component is to focus the light coming from the light source to ensure that the specimen is optimally illuminated.
The majority of the light produced by the light source is collected, and focused by the sub-stage condenser. When the condenser is correctly aligned, the specimen will be illuminated by an inverted cone of light which consequently ensures a uniform intensity and contrast in the final image.
There are two components within the sub-stage condenser which also need to adjusted for correct Koehler illumination. These are the diaphragm and centering screws.
In addition to (or in place of- in older instruments) the field diaphragm, there is also a diaphragm within the sub-stage condenser (also known as the ‘iris diaphragm’). This is adjusted by either a knurled ring around the condenser, or (more commonly) by a control lever. The purpose of the sub-stage condenser diaphragm is to adjust the angle of the cone of light which illuminates your specimen.
As I mentioned in one of my previous articles on Numerical Aperture (NA), the complete NA of the microscope system and the resolution is determined not only by the objectives, but by each of the optical components of a microscope- each of which need to be correctly aligned in relation to each other. When the diaphragm of the sub-stage condenser is opened, this results in an increase in the NA of the whole microscope and a consequent increase in resolution, contrast and illumination of the specimen on view. The centering screws of a sub-stage condenser ensure the cone of light is directly in the centre of the field of view, consequently ensuring a uniform illumination and intensity across the whole specimen image.
The five easy steps for Koehler illumination
- The first thing to do before you even start imaging is to check that the eyepieces, objectives, sub-stage condenser and the light source are all clean. I will cover microscope lens cleaning in a future article, but for the time being, always use lens cleaning tissue (not lab wipes!) and a recommended cleaning solution. Place your slide on the stage, turn the light source to the lowest setting, and then switch it on.
- With a low power objective, try to bring your specimen into sharp focus. However, if you are using a microscope which is in a shared facility, then the settings of the instrument may be completely misaligned.
Whilst looking down the eyepieces, slowly close down the field diaphragm until you see a small circle of light on your slide. - Focus the condenser next. Turn the focus wheels of the condenser slowly up and down whilst looking down the eyepieces. You will see the edges of the small circle of light go in and out of focus. In addition, you will notice a change around the circle from a red fringe to a blue fringe. Try to focus the edge to make it as sharp as possible.
- Now move your eyes away from the eyepieces and have a look at the sub-stage condenser. Here you will see the centering screws. Hold the two screws and look back down the eyepieces. When you turn the screws, you will see the circle of light moving across the field of view (horizontally and vertically). Using both of the screws, try to adjust the centering until the circle is in the middle of the field of view. After centering the condenser, slowly open the field diaphragm back up until the edge of the circle just disappears from your field of view.
- Finally, adjust the condenser (or iris) diaphragm. Look down the eyepieces and slowly move the diaphragm lever (or ring) until the ‘flare’ in your image disappears. Closing down the condenser diaphragm will increase the contrast of your specimen, although this will result in a decrease in resolution. Aim to find a balance which will be dependent on the specimen you are viewing (and your eyes).
You should bear in mind that as each objective on the microscope will have different optical properties, then you will need to set up for correct Koehler illumination when you change magnification. However, once you get used to the adjustments above, it really is just five simple steps.
To summarise: (1) close down the diaphragm, (2) focus the edges, (3) centre the spot, (4) open up the diaphragm and (5) reduce flare.
You can read an English translation of Koehler’s original 1893 paper by following this link; http://micro.magnet.fsu.edu/primer/anatomy/kohleroriginal.html
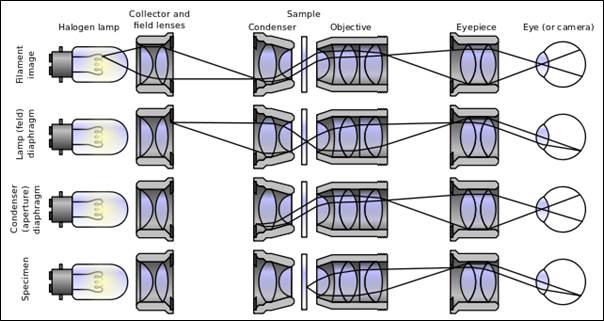
“Kohler Illumination” by Zephyris – Own work. Licensed under CC BY-SA 3.0 via Wikimedia Commons – http://commons.wikimedia.org/wiki/File:Kohler_Illumination.svg#/media/File:Kohler_Illumination.svg
AUTHOR: Martin Wilson


